New research suggests that the impact of natural and vaccine-induced immunity will be key factors in shaping the future trajectory of the global coronavirus pandemic, known as COVID-19. In particular, a vaccine capable of eliciting a strong immune response could substantially reduce the future burden of infection, according to a study by Princeton researchers(Link is external) published in the journal Science Sept. 21.

A new study led by Princeton researchers suggests that the impact of natural and vaccine-induced immunity will be key factors in shaping the future trajectory of the global coronavirus pandemic, known as COVID-19. In particular, a vaccine capable of eliciting a strong immune response could substantially reduce the future burden of infection.
“Much of the discussion so far related to the future trajectory of COVID-19 has rightly been focused on the effects of seasonality and non-pharmaceutical interventions [NPIs], such as mask-wearing and physical distancing,” said co-first author Chadi Saad-Roy(Link is external), a Ph.D. candidate in Princeton’s Lewis-Sigler Institute for Integrative Genomics(Link is external). “In the short term, and during the pandemic phase, NPIs are the key determinant of case burdens. However, the role of immunity will become increasingly important as we look into the future.”
“Ultimately, we don’t know what the strength or duration of natural immunity to SARS-CoV-2 — or a potential vaccine — will look like,” explained co-first author Caroline Wagner, an assistant professor of bioengineering at McGill University who worked on the study as a postdoctoral research associate in the Princeton Environmental Institute(Link is external) (PEI).
“For instance, if reinfection is possible, what does a person’s immune response to their previous infection do?” Wagner asked. “Is that immune response capable of stopping you from transmitting the infection to others? These will all impact the dynamics of future outbreaks.”
The current study builds on Princeton research published in Science May 18 that reported that local variations in climate are not likely to dominate the first wave of the COVID-19 pandemic and included many of the same authors, who are all affiliated with the Climate Change and Infectious Disease(Link is external) initiative funded by PEI and the Princeton Institute for International and Regional Studies(Link is external) (PIIRS).
In the most recent paper, the researchers used a simple model to project the future incidence of COVID-19 cases — and the degree of immunity in the human population — under a range of assumptions related to how likely individuals are to transmit the virus in different contexts. For example, the model allows for different durations of immunity after infection, as well as different extents of protection from reinfection. The researchers posted online an interactive version of model’s predictions(Link is external) under these different sets of assumptions.
As expected, the model found that the initial pandemic peak is largely independent of immunity because most people are susceptible. However, a substantial range of epidemic patterns are possible as SARS-CoV-2 infection — and thus immunity — increases in the population.
“If immune responses are only weak, or transiently protective against reinfection, for example, then larger and more frequent outbreaks can be expected in the medium term,” said co-author Andrea Graham(Link is external), professor of ecology and evolutionary biology(Link is external) at Princeton and an associated faculty member in PEI(Link is external).
The nature of the immune responses also can affect clinical outcomes and the burden of severe cases requiring hospitalization, the researchers found. The key question is the severity of subsequent infections in comparison to primary ones.
Importantly, the study found that in all scenarios a vaccine capable of eliciting a strong immune response could substantially reduce future caseloads. Even a vaccine that only offers partial protection against secondary transmission could generate major benefits if widely deployed, the researchers reported.
Factors such as age and superspreading events are known to influence the spread of SARS-CoV-2 by causing individuals within a population to experience different immune responses or transmit the virus at different rates. “Our models show that these factors do not affect our qualitative projections about future epidemic dynamics,” said Bryan Grenfell(Link is external), the Kathryn Briger and Sarah Fenton Professor of Ecology and Evolutionary Biology and Public Affairs(Link is external) and an associated faculty member in PEI. Grenfell is a co-senior author on the paper with C. Jessica Metcalf(Link is external), associate professor of ecology and evolutionary biology and public affairs and also a PEI associated faculty member.
“As vaccine candidates emerge, and more detailed predictions of future caseloads with vaccination are needed, these additional details will need to be incorporated into more complex models,” Grenfell said.
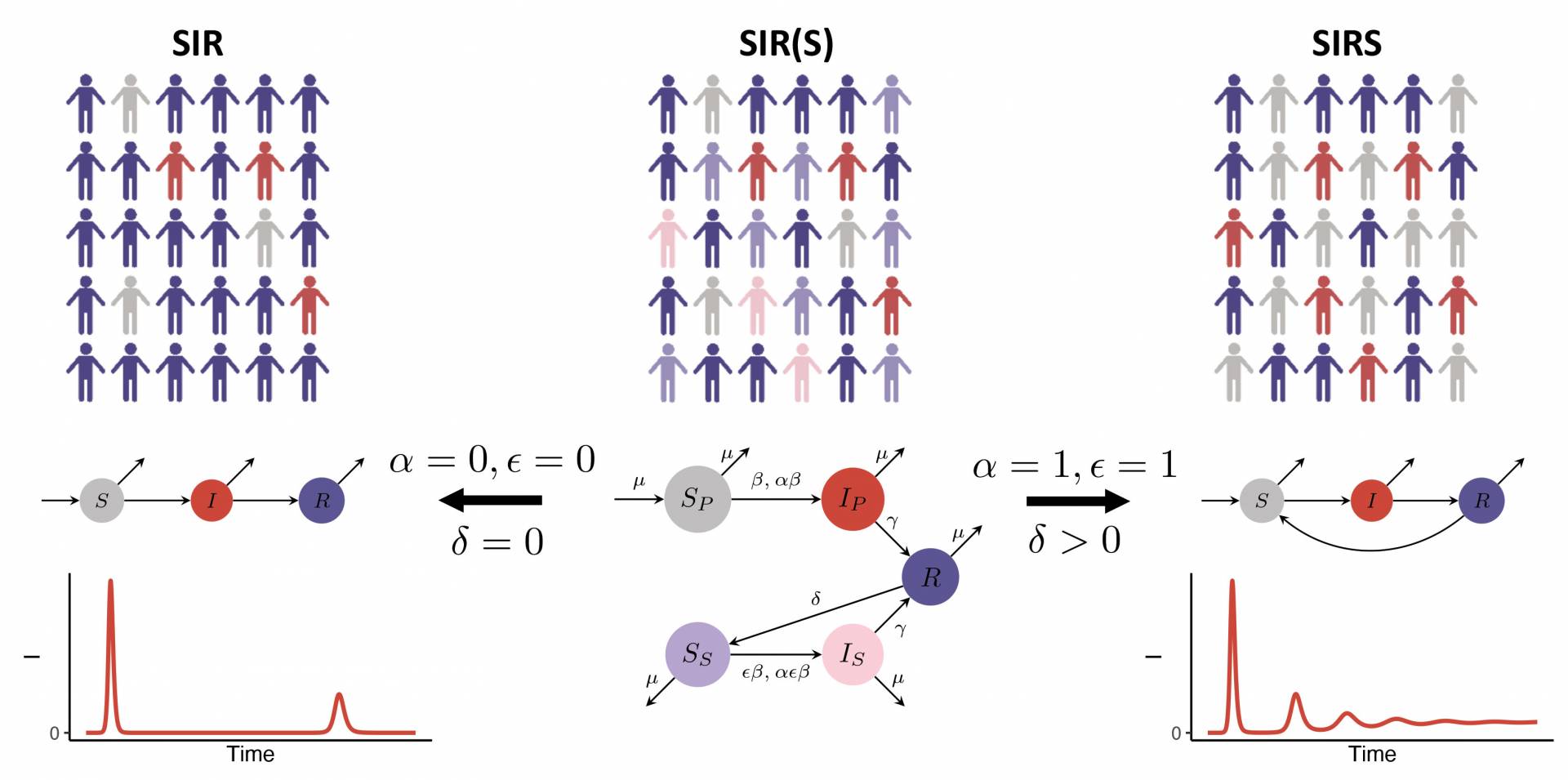
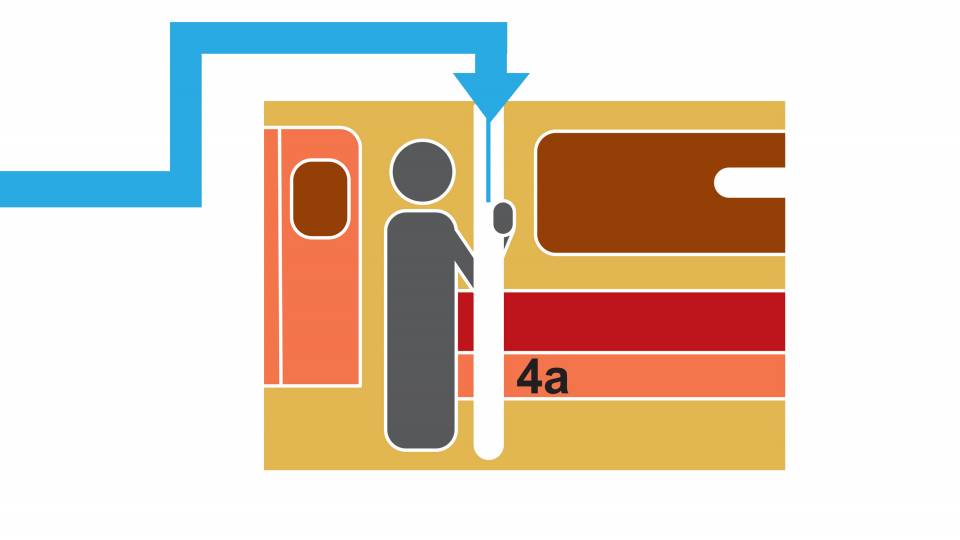
The researchers used a simple model to project the future incidence of COVID-19 cases — and the degree of immunity in the human population — under a range of assumptions on host immune responses following natural infection or vaccination. The middle flowchart (above) corresponds to the simplest model used by the researchers and allows for the incorporation of these different immune assumptions. The model found that, after the pandemic peak, a substantial range of epidemic patterns are possible as SARS-CoV-2 infection — and thus immunity — increases in the population. In all scenarios, a vaccine capable of eliciting a strong immune response could substantially reduce future caseloads.
The study authors also explored the effect of “vaccine hesitancy” on future infection dynamics. Their model found that people who decline to partake in pharmaceutical and non-pharmaceutical measures to contain the coronavirus could nonetheless slow containment of the virus even if a vaccine is available.
“Our model indicates that if vaccine refusal is high and correlated with increased transmission and riskier behavior such as refusing to wear a mask, then the necessary vaccination rate needed to reach herd immunity could be much higher,” said co-author Simon Levin(Link is external), the James S. McDonnell Distinguished University Professor in Ecology and Evolutionary Biology and an associated faculty member in PEI. “In this case, the nature of the immune response after infection or vaccination would be very important factors in determining how effective a vaccine would be.”
“When so much uncertainty in the underlying processes exists, it can be challenging to make accurate projections about the future,” Grenfell said. “We argue in this study that ultimately, a family of both simple and more complex models is the best way to proceed under these circumstances. Comparing the predictions of these models carefully and then coming up with a carefully averaged picture of the future — as with weather prediction — can be very helpful.”
One of the main takeaways of the study is that monitoring population-level immunity to SARS-CoV-2, in addition to active infections, will be critical for accurately predicting future incidence.
“This is not an easy thing to do accurately, particularly when the nature of this immune response is not well understood,” said co-author Michael Mina, an assistant professor at the Harvard School of Public Health and Harvard Medical School. “Even if we can measure a clinical quantity like an antibody titer against this virus, we don’t necessarily know what that means in terms of protection.”
“Studying the effects of T-cell immunity and cross-protection from other coronaviruses are important avenues for future work,” Metcalf said.
Additional authors on the paper include Rachel Baker(Link is external), a PEI postdoctoral research associate; Sinead Morris, a postdoctoral research scientist at Columbia University who received her Ph.D. in ecology and evolutionary biology from Princeton; and Jeremy Farrar, director of the Wellcome Trust.
The paper, “Immune life-history, vaccination, and the dynamics of SARS-CoV-2 over the next five years,” was published online by Science Sept. 21. This work was supported by funds from the Natural Sciences and Engineering Research Council of Canada, the Life Sciences Research Foundation, the Cooperative Institute for Modelling the Earth System (CIMES) at Princeton University, the James S. McDonnell Foundation, the C3.ai Digital Transformation Institute, the National Science Foundation, the US Centers for Disease Control and Prevention, and Flu Lab.