|
|
·
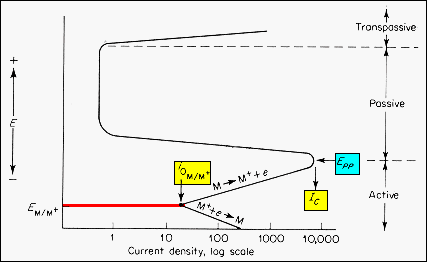
Metals
such as Fe, Cr, Ni, Ti, and their alloys demonstrate an overpotential behavior
involving active, passive, and transpassive corrosion behavior. The diagram
illustrates the behavior of these materials.
· In
the absence of an applied potential, the metal half cell is at its electrode
potential E(M/M+) and equilibrium exchange current, i0(M/M+).
As a more positive overpotential is applied, the corrosion rate increases
along the
M
-> M+ + e branch associated with a non-passivating
metal.
·
At
the condition Epp,IC,
the corrosion rate drops several orders of magnitude and due to an insulating
surface film the material is in a passive state. |