Decreasing the intake of calories and tweaking the activity of the hormone insulin are two methods long known to increase lifespan in a wide range of organisms.
In particular, studies have shown that longevity can be extended by reducing activity in the insulin-signaling pathway -- a chain of events through which insulin influences numerous biological processes, including metabolism, stress response and development.
Now, a team of Princeton biologists has found the first evidence that these mechanisms also have an impact on cognitive function.
By studying worms, the scientists were able to analyze the effects of caloric restriction and reduced insulin signaling on declines in learning and memory brought on by age. The findings have implications for the development of treatments that simultaneously help people live longer and prevent the devastating losses in memory that so often occur with age. Their results are published in the May 18 edition of the journal Public Library of Science Biology.
"The assumption in the field of longevity research has been that organisms able to live longer will function longer as well," said Coleen Murphy, an assistant professor of molecular biology and the Lewis-Sigler Institute for Integrative Genomics at Princeton, and the senior author on the paper. "It seems we need to revisit that. Different mechanisms of longevity extension may be beneficial to certain functions and detrimental to others, so it may be the case that treatments that target more than one longevity regulator will be the right approach to take."
When Murphy and her colleagues looked at the effects of caloric restriction on cognitive function in C. elegans roundworms, they found that restricting calories impaired long-term memory in early adulthood. But surprisingly, the worms did not suffer further memory reduction with age, suggesting that caloric restriction may guard against memory loss over time.
The scientists also studied worms with genetic mutations that allowed the researchers to assess separately the impact of reducing the activity in the insulin-signaling pathway. In contrast to the worms that were eating less, the worms with reduced insulin signaling demonstrated improved long-term memory performance in early adulthood and maintained learning ability better with age. However, these worms were not protected against age-related declines in long-term memory.
The team also found that molecular mechanisms underlying cognitive function in worms are the same as those previously discovered in higher organisms, including mammals, suggesting that the study has far-reaching implications.
C. elegans has long been used for research on aging and longevity, owing to the worm's simple nervous system, relatively short lifespan of just two to three weeks, and the fact that the worms experience many signs of aging. This includes reduced motility and muscle deterioration, which are seen in other organisms, including humans. Additionally, scientists conducting extensive work on the worms previously have identified several C. elegans mutants with long lifespans, which provide opportunities to explore the molecular and genetic pathways that allow these mutants to live up to 50 percent longer than normal worms. Previous research in this area includes extensive work over the past two decades by Cynthia Kenyon and her research team at the University of California-San Francisco to identify the genes that affect longevity in C. elegans. Murphy was a postdoctoral scientist in Kenyon’s lab from 2000 until 2005.
Until now, however, scientists have not known whether the roundworms experience declines in cognitive function related to age that are so often seen in humans, Murphy said. Also unknown was whether such declines, if they exist, affect mutant worms with long lifespans in the same way or on the same time scale.
In seeking to explore these questions, the researchers found themselves facing a challenge: how to you assess learning and memory in worms.
Taking a hint from Russian scientist Ivan Pavlov, famous for his early 20th-century experiments that taught dogs to associate food with the ringing of a bell, Princeton graduate student Amanda Kauffman trained worms to associate food -- in the case of C. elegans, bacteria are food -- with a chemical called butanone. The worms are not normally attracted to butanone, which smells something like a cloying combination of butterscotch and acetone, but they learned to move toward the chemical after training sessions in which they were fed in the presence of butanone.
After training, the researchers assessed learning and memory by re-exposing the worms to butanone and observing the degree to which the animals moved toward the chemical to which they normally would be averse. The biologists trained and tested worms of a variety of ages to assess the cognitive abilities of young, middle-aged and old worms.
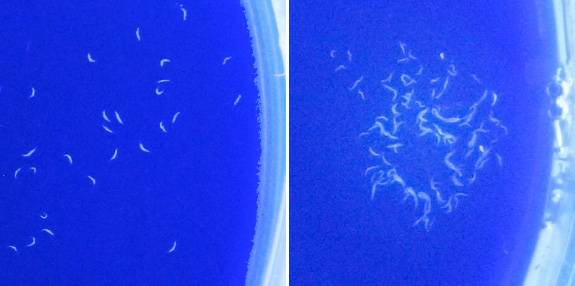
As part of an experiment to assess learning and memory in C. elegans roundworms, the image on the left shows worms that have not yet been trained to associate food with a drop of the chemical butanone that has been placed on the right side of the Petri dish. After training, the worms in the image on the right can be seen clustering around butanone that has been added. By testing normal worms as well as worms with genetic mutations that increase lifespan, the researchers were able to explore how factors that extend longevity affect learning and memory. (Photos: Coleen Murphy lab)
The number and duration of the training sessions had a marked effect on how long the worms were observed to remember the food-butanone association. The researchers found that, after a single 30-minute training session, young worms moved toward butanone when tested immediately, but they retained this short-term memory less than two hours. Following a series of seven training sessions, young worms were found to form long-term memories that lasted at least 16 hours -- a large chunk of time in the lifespan of a worm that lives only about two and a half weeks. In the average person, this would equate to remembering something for around three to six years. Further tests indicated that about half of this long-term memory faded within 24 hours, and the food-butanone association was entirely forgotten 40 hours after training. For the average person, this would equate to forgetting something after about eight to 15 years.
According to Murphy, the memory tests revealed that, relatively early in their short lives, the worms began to lose their ability to learn and retain information, with deficits starting to appear in the second day of adulthood. By the fourth day of adulthood, the worms had lost the ability to form long-term memories entirely.
After using the learning and memory tests to assess cognitive function in normal worms, the biologists did the same tests on two strains of C. elegans mutants with abnormally long lifespans.
One of the mutants tested has a defect in a gene known as daf-2, which controls the formation of the worm's insulin receptor, the researchers explained. This defect reduces the worm's ability to respond to the insulin hormone, and the gene is known to regulate survival, resistance to stress and the maintenance of motility in the worms. A similar gene in humans is known to regulate aging.
The other mutant has a genetic defect that makes it difficult for the worms to ingest food, forcing the animals to eat less. To date, caloric restriction has been observed to extend lifespan in every organism tested, including worms, mice and monkeys, Murphy said. While the reasons for this are still under investigation, scientists generally believe that the benefits of caloric restriction go well beyond preventing diseases associated with obesity, such as heart disease and diabetes, Murphy added. It appears that limiting food intake actually slows the aging process.
When the Princeton biologists conducted the learning and memory tests on the two strains of C. elegans mutants, they were surprised at the different effects on learning and memory arising from caloric restriction and reduction in the activities of the insulin-signaling pathways.
Young worms whose calories were restricted had normal short-term memories, but their long-term memories were severely impaired; the memories faded within 24 hours, as opposed to 40 hours in normal worms. The researchers determined this by analyzing the degree to which the worms moved toward the butanone in the memory tests.
But the worms did not experience a decline with age in their long-term memories, weak though they were; they were able to form long-term memories even four days into adulthood, by which time normal worms have lost this ability entirely.
In contrast, the strain of young worms with reduced activity in their insulin-signaling pathways had longer short-term memories -- lasting about six hours, or three times as long as in normal worms -- and long-term memories that lasted far longer than the 40 hours in normal worms. Additionally, these mutants retained the ability to learn much longer than normal worms. But their ability to form long-term memories faded at the same rate as in normal worms -- by day four of adulthood, the worms could no longer make long-term associations.
By looking at the molecular mechanisms at play in the worms, the biologists determined that the differences in the effects of caloric restriction and reduced insulin signaling on age-related decline in long-term memory appear to be linked to expression of a protein called CREB.
In their experiments, the researchers found that the presence of CREB, which binds to DNA and regulates the expression of genes, is crucial for the formation of long-term memories in C. elegans, but not required for learning or short-term memory. Previous studies have shown that CREB is required for long-term memory in numerous organisms, including sea slugs, fruit flies and mammals.
For example, the Princeton team observed that higher levels of CREB protein were found in young C. elegans with defective insulin receptors, which accounts for the heightened long-term memories observed in these worms. Similarly, CREB levels in the worms that were genetically altered to eat less were quite low, but they did not diminish with age. This could explain why these worms had poor long-term memories at a young age, but did not experience memory loss over time.
The research shows that different factors that extend longevity affect cognitive function in very different ways, having both positive and negative effects over the course of an organism's lifetime. While the work suggests that it may one day be possible to harness those mechanisms that protect against age-related memory loss in treatments that extend life and promote healthy brain functioning, Murphy pointed out that the results must be interpreted with a mix of caution and optimism in terms of potential applicability to the development of medical therapies for people.
"I'm optimistic because we know these longevity mechanisms in C. elegans are conserved in higher organisms, and there are reasons to believe that they could have similar effects on lifespan and cognitive function in humans," she said. "But these results also suggest that not every way of extending lifespan is good for cognitive function, which has huge implications for the development of therapies to maintain memory."
In future research, the biologists intend to examine the genetic underpinnings of learning, memory, and age-related cognitive decline in C. elegans using DNA microarray technology, Murphy said. Researchers in her lab also plan to use the worms to screen a library of chemicals known to be active in humans to assess their effects on age-related declines in cognitive function in worms.
In addition to Murphy and Kauffman, the research team included research specialist Jasmine Ashraf, graduate student Jessica Landis, and class of 2008 undergraduate Michael Corces-Zimmerman. The research was supported by the Alfred P. Sloan Foundation, the Pew Charitable Trusts, the McKnight Foundation, the W.M. Keck Foundation and the National Institutes of Health.


