|
|
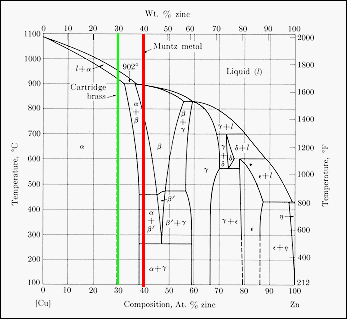
Brass
is a series of binary alloys between copper and zinc, with an upper zinc
content of about 50 wt %. The phase diagram is shown, and two brasses are
identified. Cartridge brass has the composition Cu-30 wt % Zn (green line)
and Muntz metal Cu-40 wt % Zn (red line). At room temperature cartridge
brass is a single phase substitutional solid solution of zinc in the fcc
copper structure. Muntz metal is a two phase (a
and g)
material at room temperature, both of which are solid solutions of zinc
in copper but with different compositions. When heated above about 800
C, Muntz metal becomes a single phase b-solid
solution. The presence of two phases at room temperature makes Muntz
metal less ductile that cartridge brass, but with a higher tensile strength.
Hot working of the material in the b-phase
takes advantage of the higher ductility of this single phase.
|
|
|
|
|
|
|
|
|