| Corrosion & Environmental Degradation |
|
||||||||||||||
|
|
|
|||||||||||||
| ·
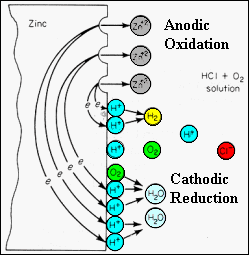
The
corrosion of zinc in an oxygenated hydrochloric acid electrolyte illustrates
the effect of multiple cathodic reactions.
· Whatever the cathodic reaction, the anodic reaction is always the oxidation of zinc: Zn -> Zn2+ + 2e. Zinc is a metallic conductor and electronic transfer to its cathodic regions is facile. ·Hydrogen ions are present in the electrolyte from dissociation of the HCl. These may react with the free electrons in the zinc metal and form hydrogen gas at the cathodic regions. 2H+ + 2e _ -> H2 (gas) · If atmospheric oxygen is present in the electrolyte, it may dissociate into oxygen atoms and react with free hydrogen ions and electrons at the zinc surface. The overall reduction process produces water molecules: O2 +4H+ +4e -> 2H2O ·The relative rates of these two cathodic reduction reactions depends on the ionic concentrations, temperature, and energy barriers for electron transfer . |
|||||||||||||||
 |
|||||||||||||||
| From:
M.G. Fontana,
"Corrosion Engineering," Wiley (1986) |
|||||||||||||||