|
·
Galvanic
corrosion cells can also be active in multi-phase alloys that have regions
with different electrode potentials.
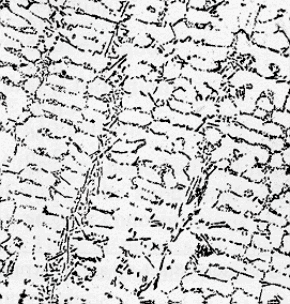
·The
photograph shows the microstructure of an eutectic aluminum-silicon alloy,
the light regions being almost pure aluminum and the dark regions almost
pure silicon. The size of the cell structure depends on the cooling rate
at which this casting-alloy was solidified.
·
The
electrochemical potential of the two regions is different, with the aluminum-
silicon alloy acting as the anode and the silicon-aluminum alloy as the
cathode. The microstructure is sub-millimetric and micro- galvanic cells
are set up in a suitable ambient.
· Similar
behavior is exhibited in iron- carbon alloys with micro-galvanic cells
acting between the ferritic and iron-carbide regions of the alloy. |
|
|
|
|
|
|
|
|
|
|
|
|