| Corrosion & Environmental Degradation |
|
||||||||||||||||
|
|
|
|||||||||||||||
| ·
To protect materials that are subject to corrosion
in a normal atmosphere, coatings of more stable materials are often used.
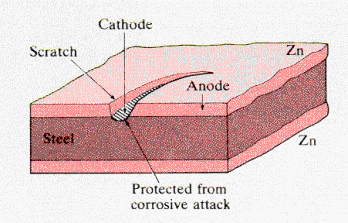
· In a moist atmosphere, a galvanic (composition) cell may be established in these multicomponent metal structures if the protective film is scratched. · The diagram shows a steel substrate with each surface plated with a layer of zinc. From their relative positions in the electrochemical series (Zn: -0.76 V, Fe: -0.44V), zinc is seen to behave as the anode and steel (iron) as the cathode. · The electrochemical reaction corrodes the anode, and, due to the large area ratio of the anode to the scratch-exposed iron, the zinc coating continues to protect the steel, which is cathodic and, therefore, not corroded. Only when the zinc is completely removed in the corrosion process will the steel will be attacked. From: Callister, "Materials Science & Engineering," Wiley (2000) |
|||||||||||||||||
 |
|||||||||||||||||
| From: Van Vlack, "Elements of Materials Science and Engineering," Addison Wesley (1985) | |||||||||||||||||
 |
|||||||||||||||||