Solids: Thermodynamics and Bonding |
|
||||||||||||||
|
|
|
|
|||||||||||||
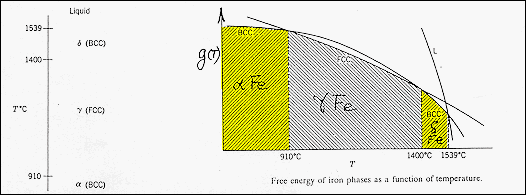
· Materials may also undergo phase transitions between different
solid structures. The diagram illustrates this for pure iron. |
|||||||||||||||
 |
|||||||||||||||
From: Brophy, Rose, & Wulff, "Thermodynamics of Structure," Wiley (1964) |
|||||||||||||||