 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Solids:
Thermodynamics and Bonding |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Electron
Distributions on Atoms
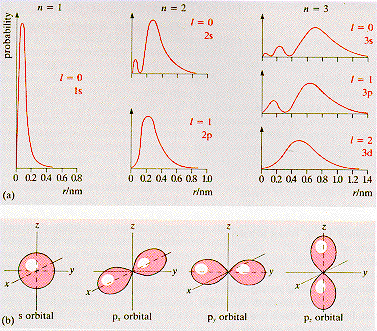
·The
electron distributions on atoms are described by wave-functions
of the types illustrated below. Each
quantum
state of an atom can accept two electrons with opposite spin.
·The
s-state is a spherically symmetric state with an angular momentum quantum
number l = 0. It can accept two electrons. The 1s state has the lowest
energy and a principal quantum number, n = 1. For the 2s and 3s states
n = 2 , 3, respectively. For each of these states, l = 0 and m = 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
·The
p-states are non-spherically symmetric and are associated with the angular
momentum quantum number, l = 1. The p-state bonds are directional
and have m = 1, 0, -1. The full p-state has a spherically symetric electron
charge distribution. |
|
|
|
|
|
|
|
|
|
|
|
|
From:
Newey and Weaver,
"Materials
Principles and Practice,"
Butterworth
(1990) |
|
|
|
|
|
|
|
|
|
|
|
