| |
The eutectoid
phase transition in the iron carbon system takes place at 727 C. The
three components in equilibrium at this temperature are Austenite, g, with
the composition Fe - 0.77 wt % C, Ferrite, a, with the composition Fe - 0.22
wt % C, and the compound Fe3C with the composition Fe - 6.7 wt % C. Austenite
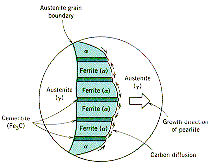
is a solid and, in general, will be polycrystalline. The formation of ferrite
and iron carbide will start at the austenite grain boundaries and the final
pearlite microstructure will have lamella of ferrite and cementite. This
is illustrated on the partial phase diagram and shown in the micrograph where
the ferrite layers are light in color. The lower diagram illustrates the phase
transition process and indicates that diffusion of carbon along the growing
boundary between the austenite and the pearlite determines its lamella structure.
The presence of interphase boundaries in pearlite and the hard-brittle
behavior of cementite makes the material less ductile than ferrite.
|
|
|
|
|
|
|
|
|
|
|