Sulfur Recovery from a Hydrocarbon Stream by a Heterogeneous Reaction with
Lead Oxide
The heterogeneous reaction of thiols with lead oxide to
form insoluble lead thiolates is the basis for a new process that separates
thiols from a hydrocarbon stream. The process uses inexpensive materials and
significantly less energy than conventional hydrodesulfurization
Process Description
The complete process for the separation of thiols from
hydrocarbons with PbO and the recovery of both the PbO and the thiols is
diagrammed below.

The hydrocarbon stream is contacted with powdered PbO
or any other lead oxide in a reactor. The PbO reacts with the thiols to form
yellow solid lead thiolates; these thiolates are insoluble in water, acetone,
cyclohexane, or toluene at temperatures below about 50�C.
PbO + 2 RSH � Pb
This reaction occurs readily at room temperature. While
the reaction can also be performed at an elevated temperature, heating to above
60-80�C causes the thiolates to melt and become miscible in hydrocarbons, preventing
their separation. If heated, the reacted stream must first be cooled to near
room temperature prior to separation, to ensure that the thiolates have
completely recrystallized. The solid thiolates can either be filtered or
settled to achieve the desired separation.
Once the thiolates are separated from the hydrocarbon
mixture, they can be converted back to the original thiols and a Pb salt by
reactive extraction with dilute nitric acid.
Pb
The Pb
The compound Pb
The two liquid phases, now consisting of an aqueous
lead salt solution and a hydrophobic thiol layer, are allowed to settle and are
separated. The aqueous layer is separated and evaporated off, leaving solid Pb
An alternative method for desulfurizing stocks of
petroleum fuels is to use this reaction with metallic Pb as the PbO source.
Metallic Pb oxidizes rapidly when exposed to humid air. The natural oxide
coating on a piece of metallic Pb is also active for this reaction, provided
that a supply of oxygen, such as air, is available to regenerate the surface
oxide layer. The reactor and separator in Figure 1 could be replaced by rods of
Pb placed into storage tanks of thiol-containing petroleum fractions.
Results
The reaction of PbO with excess n-octanethiol
produced thiolates at room temperature with or without mild agitation. The
yield of thiolates, as determined by the change in mass of the solids divided
by the theoretical change in mass to convert all PbO into Pb
The limit for the removal of thiols by this reaction
can be determined from thermodynamics. The equilibrium constant for this
reaction, assuming unit water activity
Figure 2 presents a series of
photographs demonstrating the reaction of 3 mL n-octanethiol diluted
with 4 mL cyclohexane with 0.75g PbO2 at room temperature, without
agitation. PbO2 was used in the photographs for color contrast
purposes, since the massicot form of PbO and the thiolate product are both
yellow. PbO2 is black but reacts to form the same yellow thiolate
product. Both compounds react in the same way. The reaction is marked by a
rapid growth in apparent solids volume as the liquid thiol is converted into
solid thiolate. The color change from the black PbO2 to the yellow PbO
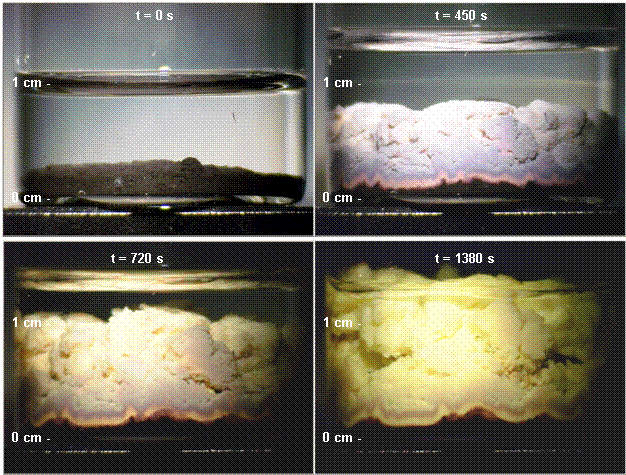
Figure 2. Photographs
of the reaction of PbO2 with n-octanethiol.
Photographs are chronological, starting from top left, occurring at elapsed
times shown. The initial mixture was 0.75 g PbO2, 4 mL cyclohexane,
and 3 mL n-octanethiol
The reaction of n-octanethiol with metallic Pb
wire was also successful. An example of this reaction is shown in Figure 3 in
which a piece of Pb wire is placed into a liquid consisting of 2 mL cyclohexane
and 4 mL of n-octanethiol. The reaction is much slower than the reaction
with powdered PbO due to the time required for oxygen to diffuse to the Pb
surface; the Pb wire also had a lower surface area compared to the powdered
oxides. The reaction shown in the photographs occurred over a period of about
62 hours. The thiolates that were formed on the Pb wire grew in the form of
plates which were pushed outwards as new thiolates were produced at the Pb
surface.
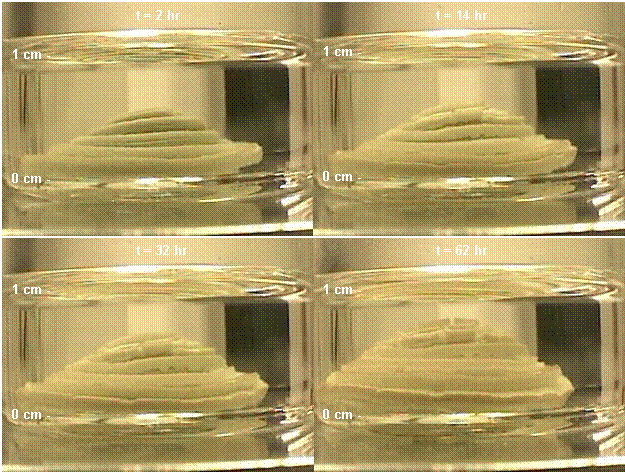
Figure 3. Photographs of the
reaction of n-octanethiol and metallic Pb wire. Initial conditions are a
coil of Pb wire
In the extraction studies, the Pb thiolates were
successfully decomposed back to the original thiols and Pb