Organic Vapor Phase Deposition for Optoelectronic Devices
The
growth of molecular organic thin films by the process of organic vapor phase
deposition (OVPD) was initiated by Professor Steve
Forrest OVPD transports organic molecules to a
cold substrate by a hot inert carrier gas. It has proven useful for the
deposition of organic semiconductors, and is capable of patterned growth with
micron resolution. Professors Benziger and Forrest collaborated to
engineering the process for a scalable continuous process. Most recently, Profs. Forrest and Benziger have
demonstrated direct printing of molecular organic materials based on a new
method of growth, organic vapor jet printing (OVJP),
where molecules are carried to the substrate by a hot carrier gas jet.
The diameter of the deposit is limited by the gas jet dynamics and nozzle dimensions,
and is capable of generating patterns ~500nm.
The context and concept of OVPD: Background and Related Work
Typically, thin (<100nm) film
molecular organic electronic devices such as organic light emitting devices (OLEDs), are grown by vacuum thermal
evaporation (VTE), permitting the high degree of
purity, pattern, and structural control needed for high performance operation.
However, control of film thickness uniformity and dopant
concentrations over large areas needed for many applications can be difficult
when using vacuum evaporation – currently the most commonly used technique for
the deposition of organic molecular solids. In addition, a considerable
fraction of the evaporant coats the cold walls of the
deposition chamber; over time, inefficient materials use results in a thick
coating which can flake off, leading to particulate contamination of the system
and substrate. The potential throughput for vacuum evaporated organic
thin film devices is low, resulting in high production costs. Low
pressure organic vapor phase deposition (OVPD) has
been demonstrated as an alternative technique that significantly improves
control over doping, and is adaptable to rapid, particle-free, uniform
deposition of organics on large-area substrates.
Carrier Gas Flowrate ( )
Kinetic
Equilibrium
Temperature (Tcell)
Equilibrium
T
Constant
a)
b)
Fig.
1: (a) Schematic of the OVPD process, (b)
the regimes of OVPD growth.
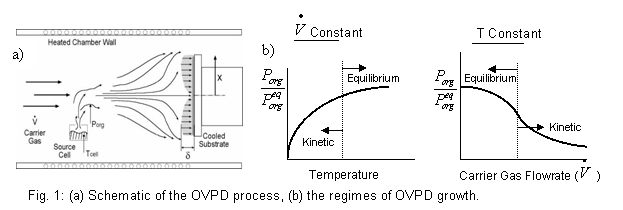
In OVPD, the
organic compound is thermally evaporated into a diluting, non-reactive gas
stream, and then transported in a hot-walled reactor toward a cooled substrate
where condensation occurs (see Fig. 1). Flow
patterns may be engineered to achieve a substrate-selective, uniform
distribution of organic vapors, resulting in a very uniform coating thickness
and minimized materials waste. Virtually all of the organic materials
used in thin film devices have sufficiently high vapor pressures to be
evaporated at temperatures below 400°C, and then be transported in the vapor
phase by an inert carrier gas such as nitrogen. This allows for
positioning of evaporation sources outside of the reactor tube spatially separating the functions of evaporation and
transport, thus leading to precise control over the deposition process.
To grow doped films with uniform composition across the entire substrate area,
the component streams must be mixed prior to deposition. By carrying out
the process at reduced pressure, gas diffusivity is increased, improving rates
of mass transfer between component streams and to the substrate, which promotes
thickness uniformity of the deposited films.
In vapor phase deposition, organic molecules are carried out
of the source cell at a rate, r, proportional to the volumetric flow velocity
of the carrier gas,  ,
and the concentration of organic vapors in the evaporation cell, Porg/RTcell:
,
and the concentration of organic vapors in the evaporation cell, Porg/RTcell:
 (1)
(1)
Here,
Tcell is the evaporation cell temperature,
Porg is the actual vapor
pressure of the organic material, and R is the universal gas constant. It
can then be shown[7] that the partial pressure of
the organic evaporant in the carrier gas stream is
given by:
 ,
(2)
,
(2)
where kevap and kcond are kinetic factors proportional to the
rates of evaporation and condensation, respectively, and  is
the equilibrium vapor pressure of the organic material. As Eq. (2) and Fig. 1b show, at
high evaporation temperatures and sufficiently low gas flow rates, the vapor
and solid in the source region equilibrate (i.e.
is
the equilibrium vapor pressure of the organic material. As Eq. (2) and Fig. 1b show, at
high evaporation temperatures and sufficiently low gas flow rates, the vapor
and solid in the source region equilibrate (i.e.  ),
and the concentration of organic exiting the source is constant. The
resulting flux of organic species in the “equilibrium” evaporation regime is
thus proportional to the vapor pressure and the carrier gas flow rate. At
the other extreme of low evaporation temperatures and high gas flows, the
carrier gas sweeps the organic out of the source region as quickly as it
evaporates, forcing the system away from equilibrium. In this case, the
concentration of organics in the gas stream is proportional to revap and inversely proportional to
),
and the concentration of organic exiting the source is constant. The
resulting flux of organic species in the “equilibrium” evaporation regime is
thus proportional to the vapor pressure and the carrier gas flow rate. At
the other extreme of low evaporation temperatures and high gas flows, the
carrier gas sweeps the organic out of the source region as quickly as it
evaporates, forcing the system away from equilibrium. In this case, the
concentration of organics in the gas stream is proportional to revap and inversely proportional to  .
In the “kinetic” evaporation regime, therefore, the flux of organic material
leaving the source is independent of the carrier gas flow. Figure 1b
illustrates how the vapor pressure of organics exiting the source varies with
temperature and flow rate for both the equilibrium and kinetic evaporation
regimes. It will become apparent below that the ability to operate OVPD
in either of these two regimes provides unprecedented
opportunities to control the structure and quality of the thin film.
.
In the “kinetic” evaporation regime, therefore, the flux of organic material
leaving the source is independent of the carrier gas flow. Figure 1b
illustrates how the vapor pressure of organics exiting the source varies with
temperature and flow rate for both the equilibrium and kinetic evaporation
regimes. It will become apparent below that the ability to operate OVPD
in either of these two regimes provides unprecedented
opportunities to control the structure and quality of the thin film.
As
in vacuum evaporation, the equilibrium vapor pressure of the organic material
depends exponentially on cell temperature, Tcell.
The partial pressures of the evaporant leaving the
source region are:
 (3)
(3)
 ,
(4)
,
(4)
where DHvap is the enthalpy of vaporization specific to
each compound, and superscripts “eq” and “kin” denote
equilibrium and the kinetic evaporation modes, respectively.
Hence,
a significant difference between vacuum evaporation and OVPD is that in the
latter process, the rate of introduction of the organic species onto the
substrate is determined by both source temperature (where the
rate is exponential with temperature) and gas flow rate,  (providing
linear control of source introduction, c.f. Eq. (5)).
Hence, we have shown doping of one or more organic species into a host thin
film is far more controllable than using simple temperature control
characteristic of vacuum deposition. We have demonstrated very low and
controllable doping concentrations of <0.5% of
a red lumophore, DCM2, doped into an Alq3
host.
(providing
linear control of source introduction, c.f. Eq. (5)).
Hence, we have shown doping of one or more organic species into a host thin
film is far more controllable than using simple temperature control
characteristic of vacuum deposition. We have demonstrated very low and
controllable doping concentrations of <0.5% of
a red lumophore, DCM2, doped into an Alq3
host.
Temperature
probes
To
Pump
Rotating
cooled holder
Thickness
monitor
4-zone heater
Glass
chamber
4
source
barrels
Mechanical
Shutter
Carrier
Gas
Inlets
Fig.
2: Schematic of original OVPD system. Photo shows the system with the clam-shell
furnace open to show a clean glass tube and substrate holder after >150
growth runs

Organic Vapor Jet Printing (OVJP)
OVJP
is a natural extension of OVPD deposition through shadow mask apertures, where
organic patterns are individually deposited through a small orifice onto a
substrate located directly beneath a nozzle. The nozzle itself is fed by
a vapor of organics and a carrier gas, thereby transporting a small amount of
material in a spatially confined area. Similar to ink jet printing,
the deposition of individual pixels on extended plastic substrates continuously
deployed in close proximity to the localized jet of gas can be achieved.
It differs substantially from solution-based ink jet printing of polymers,
however, in that the solvent in OVJP is a gas. Hence, it is easily
volatilized during growth to leave a uniform film of the desired organic.
This process, invented at Princeton,
has the possibility of revolutionizing the growth of small molecule organic
thin films by rapidly and simply depositing ultrasmall
(nanometer scale) patterns of organic thin film
materials or precursors. Like OVPD, the process works by passing a heated
gas through a hot organic source powder or liquid. The gas then entrains
the molecular species, carrying it through a valved
nozzle to a cooled, horizontally translating substrate where the material
deposits. The ultimate deposit resolution achievable by this method
is determined by the distance from the nozzle to the substrate, the gas flow
velocity, the background pressure, temperature, and the diameters of the
orifice and the tip. The practical limits to these dimensions are ~500nm,
using an orifice with micropores. Below this
diameter, the gas viscosity will limit the transport of material through the
orifice.
mixing
chamber
N2
N2
N2

Schematic of the OVJP process. Carrier gases pick up
the organic vapor and direct the organic onto the substrate through a
nozzle. The higher mass organic retains its forward momentum towards the
substrate and is kept collimated to provide good resolution.

Organic Vapor Jet Printing Apparatus.
1.5mm
HPTM
2250 ColorJet
Ink
Jet Printer
SPTM
2000
Organic
Vapor Jet Printer
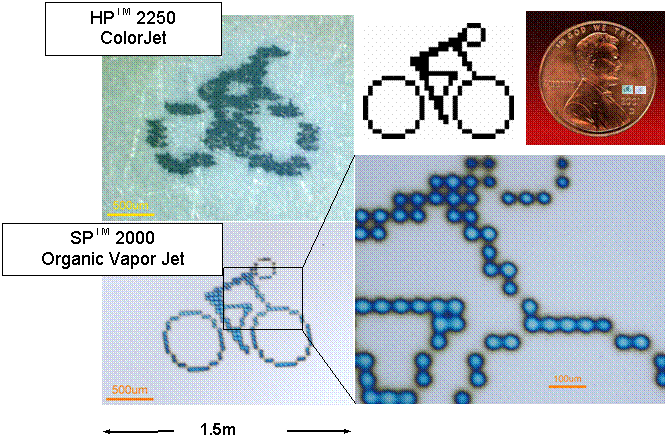
Example of the resolution of OVJP. The resolution of
the prototype system is ~ 1200 dpi.
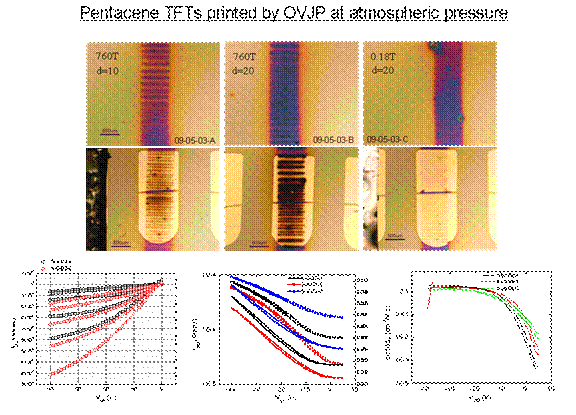
OVJP
can print thin film transistors without the need for a vacuum system.

![]() ,
and the concentration of organic vapors in the evaporation cell, Porg/RTcell:
,
and the concentration of organic vapors in the evaporation cell, Porg/RTcell:![]()
 ,
,
![]() is
the equilibrium vapor pressure of the organic material. As Eq.
is
the equilibrium vapor pressure of the organic material. As Eq. ![]() )
)![]() .
In the “kinetic” evaporation regime, therefore, the flux of organic material
leaving the source is independent of the carrier gas flow. Figure 1b
illustrates how the vapor pressure of organics exiting the source varies with
temperature and flow rate for both the equilibrium and kinetic evaporation
regimes. It will become apparent below that the ability to operate OVPD
in either of these two regimes provides unprecedented
opportunities to control the structure and quality of the thin film.
.
In the “kinetic” evaporation regime, therefore, the flux of organic material
leaving the source is independent of the carrier gas flow. Figure 1b
illustrates how the vapor pressure of organics exiting the source varies with
temperature and flow rate for both the equilibrium and kinetic evaporation
regimes. It will become apparent below that the ability to operate OVPD
in either of these two regimes provides unprecedented
opportunities to control the structure and quality of the thin film.![]()
![]() ,
,
![]()




