The Qualitative Analysis scheme test for the presence of free ions in solution. These test are designed to give a color change or formation of a precipitate when the ion being tested is present. In order to recognize a positive test you performed a set of standard tests on solutions that contained either iron or oxalate ions. You should write balanced equations for Test 1, 2, 3, and 5 and note any color change or precipitate formation. And test 4, the flame test, is testing for potassium.
Oxalate Test: Test 1 and 3 are tests for the oxalate anion (C2O42-).
Test 1 - Ca(NO3)2 solution.
![]()
Test 3 - KMnO4 solution. This is a redox reaction and was the chemistry behind the lab practical exam last semester.
![]()
Iron Test: Test 2 and 5 are testing for the presence of iron in either the 2+ or 3+ oxidation state.
Test 2 - Sulfosalicylic acid. This is a test for Fe3+ only. The ratio of iron : sulfosalicylic acid in the colored complex formed is 1:1 (Sulfosalicylic acid is abbreviated as SSA)
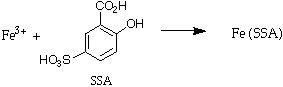
Test 5 - Aqueous ammonia solution. You performed this test on both Fe2+ and Fe3+ ions. The results from this text will allow you to later determined the oxidation state of the iron in the original complex formed.
![]()
![]()
Test 4 - Flame test. Report the color of the flame for potassium.
Include your results from theTest on the Compound and Test on the Compound After Base Treatment. Remember that the test will be positive for free ions in solution.
Compare the color of the precipitate after base treatment to the result from standard test 5 to determine the oxidation state of iron in the complex.