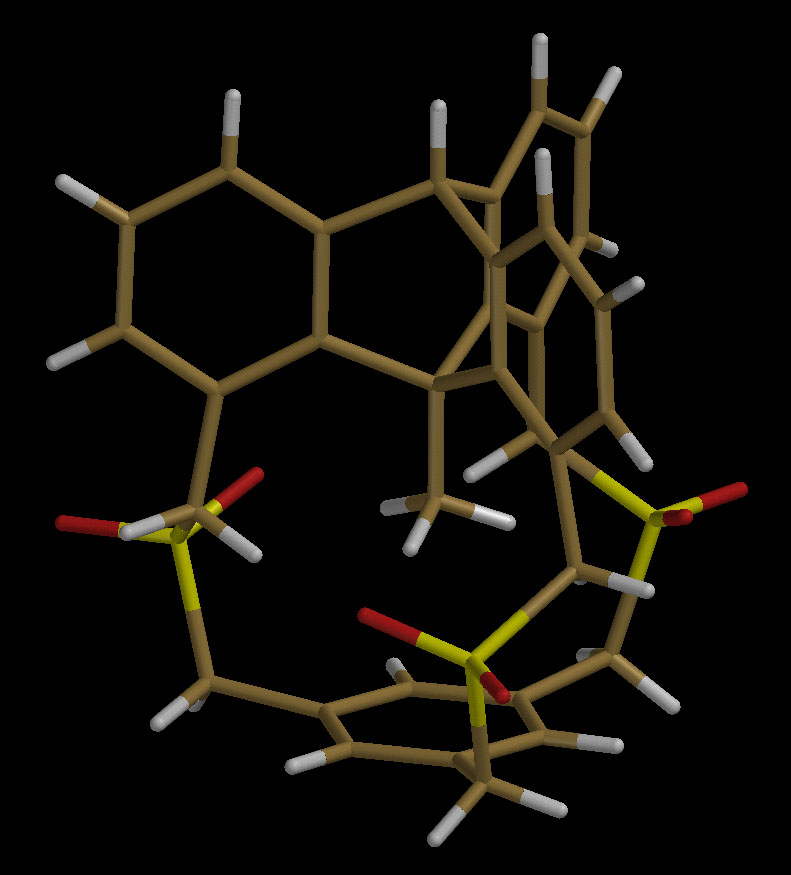
An In-Methylcyclophane
This in-methylcyclophane is the first molecule to have a methyl group
projected directly toward the center of an aromatic ring. Several previous
attempts to prepare in-methylcyclophanes led only to the formation of the
out-isomers due to the flexibility of the methyl-bearing caps. In this
case, the methyl is attached to a rigid triptycene moiety so that out-isomer
formation is completely suppressed. The in-methyl protons and carbon show
high-field NMR resonances, as expected, and the in-methyl carbon atom is
only 2.88 A from the center of the basal benzene ring. However, the
molecule's most remarkable feature is the compression of the C-Me bond
distance to 1.48 A from the 1.54 A average observed in similar, but
uncompressed methyltriptycenes and triarylethanes. For a brief
communication describing the synthesis and structure of this compound,
see "Sterically Congested in-Methylcyclophanes," Q. Song, D. M. Ho, and
R. A. Pascal, Jr., J. Am. Chem. Soc. 2005, 127, 11246-11247.