|
·
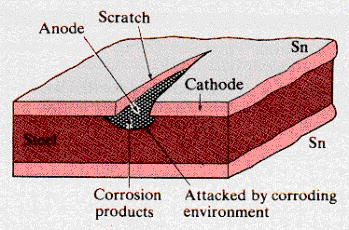
Tin-plating is an alternate mode of protecting steel.
The diagram shows a scratched section of the tin (Sn) steel (Fe) structure.
·The
position of tin and iron in the electro-chemical series indicates that
tin (-0.14V) will act as the cathode and iron (-0.44V) as the anode in
the local galvanic cell.
·
For
this system, the anodic area is much smaller than the cathodic coating.
Corrosion of the steel anode will occur, the electrolytic film will penetrate
the crack, and continuing corrosion will under-cut the tin layer. Corrosion
products deposited in this region may slow the reaction rate. In many cases,
rapid local corrosion will cut completely through the thickness of the
steel.
·
Tin
is only a protective coat for an iron substrate if it is scratch free! |
|
|
|
|
|
|
|
|
|
|
|
|